Legume host plants support reproduction of nitrogen-fixing rhizobia bacteria in their root nodules and replenish soil populations when nodules senesce (Kuykendall et al., 1982). Even without host plants, rhizobia populations can survive in soil for 17 years or more (Narozna et al., 2015). But is that population-level survival due to reproduction of rhizobia using soil resources, balancing death of cells in the soil? Or could individual rhizobia cells survive in soil for years or decades?
During symbiosis, a rhizobia cell can accumulate the lipid, polyhydroxybutyrate (PHB), which they may use as an energy source after returning to the soil. Katherine Muller measured PHB levels in rhizobia cells in soybean nodules and calculated that PHB could last for many years in soil, if rhizobia are sufficiently dormant (Muller & Denison, 2018), which they may be (Ratcliff & Denison, 2011).
What if rhizobia strains differ in how they allocate carbon (from their plant host) between PHB accumulation versus respiration supporting nitrogen fixation? Could strains that hoard more PHB survive longer in the soil? How much longer?
To find out, Katherine (now Dr. Muller) collected soil from field plots (at the University of Minnesota’s Southern Research and Outreach Center) that differed in how often soybeans were grown. She inoculated plants in a greenhouse with soil from the different plots (a “common-garden” experiment) and measured PHB levels in rhizobia in the resulting root nodules. Her results have just been published in Agronomy Journal (Muller & Denison, 2024).
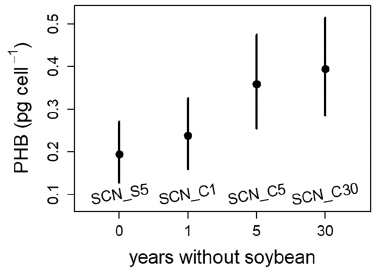
The rhizobia strains that survived longest (5 or 30 years without soybean) were those that, on average, accumulated twice as much PHB while in nodules. You wouldn’t expect this difference if accumulating more PHB in a nodule just let a rhizobia cell survive for 6 months in the soil rather than 2 months. The 6-month survivors would have more opportunities to reproduce (by dividing) before they get eaten by an protozoan, or something. But each cell division would leave the next generation with half as much PHB. All the original PHB would be gone long before the 5-year mark. So these data seem more consistent with the hypothesis that Ratcliff and Muller developed earlier, namely, that individual rhizobia cells can go dormant and survive in soil for years, if they start with enough PHB.
An alternative explanation would be that alleles that increase PHB accumulation in nodules also increase PHB accumulation in soil, perhaps during short-term periods when carbon resources are available. So, are there other ways to tell whether rhizobia in soil are mostly active or dormant? I would expect a population with significant turnover (reproduction and death) to accumulate more mutations, both from the reproductive process itself and from selection acting more on active than on dormant cells. So it’s interesting that after 17 years in the soil, rhizobia “strains were undistinguishable from the original inoculum strains” (Narozna et al., 2015).
A possible application of our research is to identify soybean genotypes that impose stricter “sanctions” (Kiers et al., 2003; Oono et al., 2011) on mediocre rhizobia strains, thereby enriching the soil with more-beneficial, locally-adapted strains (Denison & Muller, 2022). This might not work if the most-beneficial strains allocate so little carbon to PHB that they starve before the next soybean crop. So it’s encouraging that the graph above suggests that even lower-PHB rhizobia can survive for a year or so without a plant host. Stricter host-imposed sanctions might therefore benefit the next soybean crop in current two-year rotations, although stricter sanctions might be less beneficial in longer rotations, which might otherwise be more sustainable.
References
Denison, R., & Muller, K. (2022) An evolutionary perspective on increasing net benefits to crops from symbiotic microbes. Evolutionary Applications 15: 1490-1504.
Kiers, E.T., Rousseau, R.A., West, S.A., & Denison, R.F. (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425: 78-81.
Kuykendall, L.D., Devine, T.E., & Cregan, P.B. (1982) Positive role of nodulation on the establishment of Rhizobium japonicum in subsequent crops of soybean. Current Microbiology 7: 79-81.
Muller, K.E., & Denison, R.F. (2018) Resource acquisition and allocation traits in symbiotic rhizobia with implications for life-history outside of legume hosts. Royal Society Open Science 5: 181124
Muller, K., & Denison, R. (2024) Prolonged soybean absence in the field selects for rhizobia that accumulate more polyhydroxybutyrate during symbiosis. Agronomy Journal https://doi.org/10.1002/agj2.21578
Narozna, D., Pudelko, K., Kroliczak, J., Golinska, B., Sugawara, M., Madrzak, C.J., & Sadowsky, M.J. (2015) Survival and competitiveness of Bradyrhizobium japonicum strains twenty years after introduction into field locations in Poland. Applied and Environmental Microbiology 81: 5552-5559.
Oono, R., Anderson, C.G., & Denison, R.F. (2011) Failure to fix nitrogen by non-reproductive symbiotic rhizobia triggers host sanctions that reduce fitness of their reproductive clonemates. Proceedings of the Royal Society, London B 278: 2698-2703.
Ratcliff, W.C., & Denison, R.F. (2010) Individual-level bet hedging in the bacterium Sinorhizobium meliloti. Current Biology 20: 1740-1744.